News
New Catalyst for Ethanol Fuel Cells Shows Promise for Liquid-Fuel-Cell-Powered Drones
Scientists are working to develop ethanol based fuel for liquid-fuel-cell-powered drones that are lighter and hence more efficient.
“Ethanol fuel cells are lightweight compared to batteries. They would provide sufficient power for operating drones using a liquid fuel that’s easy to refill between flights–even in remote locations,” notes Jia Wang the Brookhaven Lab chemist who led the work.
A new ‘Journal of the American Chemical Society’ research paper described the catalyst as the key component with the property of steering the electro-oxidation of ethanol down an ideal chemical pathway that releases the liquid fuel’s full potential of stored energy.
Scientists Radoslav Adzic, Zhixiu Liang, Jia Wang, Eli Stavitski, and Liang Song at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and the University of Arkansas have developed a highly efficient catalyst for extracting electrical energy from ethanol, an easy-to-store liquid fuel that can be generated from renewable resources.
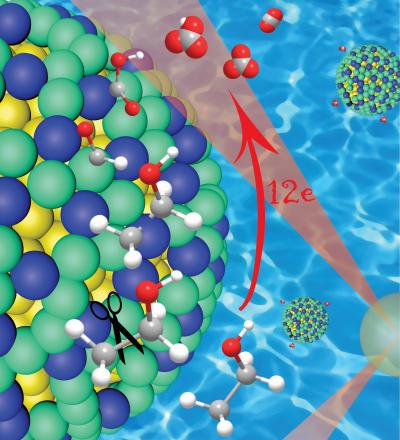
A close-up of the platinum/iridium (green/blue) shell over a gold nanoparticle core (yellow), showing how this catalyst cleaves the carbon-carbon (gray) bonds in ethanol while initially leaving hydrogen atoms attached. The hydrogen protects the carbon in the early stages of the reaction, preventing the formation of catalyst-poisoning carbon monoxide, which enables complete oxidation and the release of 12 electrons.
“This catalyst is a game changer that will enable the use of ethanol fuel cells as a promising high-energy-density source of ‘off-the-grid’ electrical power,” added Jia Wang. One particularly promising application: liquid fuel-cell-powered drones.
Much of ethanol’s potential power is locked up in the carbon-carbon bonds that form the backbone of the molecule. The catalyst developed by Wang’s group reveals that breaking those bonds at the right time is the key to unlocking that stored energy. The new catalyst–which combines reactive elements in a unique core-shell structure that Brookhaven scientists have been exploring for a range of catalytic reactions–speeds up all of these steps.
Jingyi Chen of the University of Arkansas, who was a visiting scientist at Brookhaven during part of this project, made the catalyst by a synthesis method to co-deposit platinum and iridium on gold nano particles. The platinum and iridium form “mono-atomic islands” across the surface of the gold nano particles. Chen explained that this arrangement is the key that accounts for the catalyst’s outstanding performance.
“The gold nanoparticle cores induce tensile strain in the platinum-iridium mono-atomic islands, which increases those elements’ ability to cleave the carbon-carbon bonds, and then strip away its hydrogen atoms,” Chen said.
Zhixiu Liang, a Stony Brook University graduate student and the first author of the paper, performed studies in Wang’s lab to understand how the catalyst achieves its record-high energy conversion efficiency revealed, “The spectra revealed that the new catalyst steers ethanol toward the 12-electron full oxidation pathway, releasing the fuel’s full potential of stored energy.”
As per Wang the next step is to engineer devices that incorporate the new catalyst. The mechanistic details revealed by this study may also help guide the rational design of future multi-component catalysts for other applications. This work was funded by the U.S. Department of Energy’s Office of Science and the National Science Foundation.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy which is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of modern time.
Citation: Direct 12-Electron Oxidation of Ethanol on a Ternary Au(core)-PtIr(Shell) Electrocatalyst, Zhixiu Liang, Liang Song, Shiqing Deng, Yimei Zhu, Eli Stavitski, Radoslav R. Adzic, Jingyi Chen Jia X. Wang, J. Am. Chem. Soc.2019 https://doi.org/10.1021/jacs.9b03474